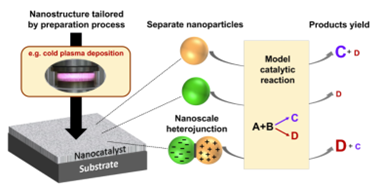
Solid catalysts, often nanoscale materials, are at the heart of many technologies. Due to their unique structure, they play a crucial role in accelerating chemical reactions, which is essential in industrial processes such as fuel production, chemicals, pharmaceuticals, and environmental protection. The ability to precisely design their molecular structure allows for tailoring catalysts to specific reactions, leading to more sustainable and economical technological processes. In a recent article by the team from the Department of Molecular Engineering titled "Classical Concept of Semiconductor Heterojunctions in the Approach to Nanohybrid Catalysts" (J. Tyczkowski and H. Kierzkowska-Pawlak), the authors delve into the secrets of nanohybrid catalysts—catalysts composed of nanoparticles or nanolayers with different molecular structures. The paper explains the role of electronic interactions in such nanosystems, often enigmatically referred to as "synergy," which determine their catalytic activity in thermochemical conversion processes. This new approach to the "alchemy" of catalysts demonstrates that creating heterojunctions—areas filled with electric charges at the contact boundary between nanoparticles or nanolayers—can fill their entire volume, significantly affecting catalytic properties.
For example, consider two distinct semiconductor materials: cobalt oxide and iron oxide. Cobalt oxide nanoparticles are highly active in producing methane from carbon dioxide and hydrogen, while iron oxide nanoparticles perform much weaker. However, if we create a nanohybrid of these two oxides, it will catalyze the reaction of carbon dioxide and hydrogen into carbon monoxide, which—like methane—is a useful industrial substrate. In the resulting nanohybrid, cobalt oxide nanoparticles are positively charged, while iron oxide nanoparticles are negatively charged, which explains the change in catalytic performance when referring to active center theory.
Graphical abstract: Cold plasma as a tool for synthesizing nanohybrid catalysts is a key discovery—connected nanoparticles of two semiconductors (the nanohybrid) are filled with electric charges, causing them to behave very differently in contact with reagents compared to isolated ones. Source: ACS Appl. Mater. Interfaces 2024, 16, 29, 37339-37345.
Thanks to a deeper understanding of interactions between different semiconductors and the accompanying charge flow phenomena across the junction, it will be easier to control the catalytic properties of such systems and develop new, more efficient catalysts for a wide range of industrial applications such as CO₂ hydrogenation, catalytic converters, or biomass conversion.
The new approach by scientists from Lodz University of Technology to nanohybrid catalysts has been commissioned and published as a "Perspective" in the journal "ACS Applied Materials & Interfaces," also featuring on its cover. Although the proposed concept is still in its early research stages, it could have a significant impact on developing more effective nanohybrid catalysts tailored to specific needs—explain the authors. Basic research in this area is also conducted by Bartosz Panek (PhD student at IDS TUL), and the results obtained are very promising.
It is worth noting that the team from the Department of Molecular Engineering has already developed an effective method for producing nanohybrid catalysts based on thin film deposition in cold plasma. Previous research by the entire team has shown that plasma technology enables designing both molecular structure and nanostructure of thin-film catalysts based on inexpensive and readily available transition metals such as iron, nickel, or cobalt. The thin films produced can be applied to any supports, for example, in the form of fine meshes, allowing for creating sophisticated catalytic packings with precisely designed geometry. This is crucial for efficiently implementing catalytic processes on an industrial scale.
The results presented in this article for nanohybrid materials confirm the significant role that interfacial electronic interactions play in controlling catalytic activity and selectivity in selected thermochemical conversion processes involving CO₂. There is already justified hope for a much broader application of this concept and its realization in many other thermochemical catalysis processes.